
Epigenetic alterations allow for adaptation in response to environmental influences. For example, if a pregnant person experiences severe stress, it can potentially lead to epigenetic changes that may alter how stress responses are expressed in the fetus and later in life.iv,v The combined influence of genetics and environment on an individual’s epigenome can have long-term effects on an individual’s health.vi These effects may extend into adulthood, potentially impacting certain traits and/or susceptibility to some conditions.vii
The use of donor eggs, instead of one’s own eggs, in IVF can be a sensitive issue and there are often questions about biological impacts of the donor egg recipient. There are many ways to build families, and it is important that donor egg recipients appreciate that their role in their child’s development extends far beyond the child’s DNA sequence.
What is epigenetics?
Our DNA contains over 20,000 genes. The specific DNA sequence in our genes determines our traits and characteristics. Epigenetics is the study of how our behaviors and the environment in which we live can affect the way our genes are interpreted without changing the actual DNA sequence.viii The term “epigenetics” originates from the Greek word "epi" meaning “over” or “above,” indicating an influence over the genome.
One can think of epigenetic marks as chemical molecules that act as “tags” or ”labels” on our genes. Therefore, the “epigenome” is the set of all these tags and acts as instructions to determine when genes are turned on or off.ix Epigenetic tags do not change the gene’s DNA sequence, but rather influence how genes are read or “expressed,” impacting a wide range of the body's functions and development.x During fetal development, epigenetic tags can be added or removed in response to external factors such as nutrition, stress, or exposure to harmful chemicals.xi
Types of epigenetic modifications
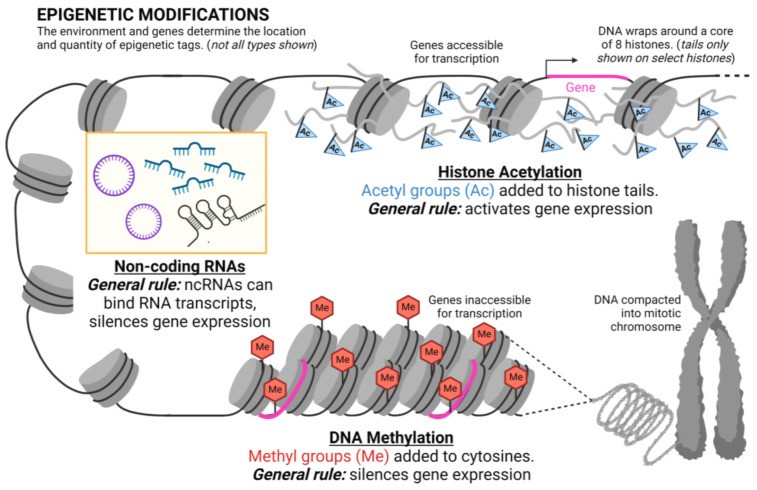
There are several types of epigenetic modifications (Figure 1), including DNA methylation, histone acetylation, and non-coding RNA.
These epigenetic mechanisms are dynamic.xii Unlike changes to the DNA sequence, which are typically permanent, many epigenetic modifications are reversible.xiii Their reversibility is a crucial aspect of epigenetics and suggests the potential for therapies to "reset" epigenetic marks to address specific conditions or diseases. However, the complexity of the process means that reversibility may vary based on the disease and type of epigenetic modification.xiv
DNA methylation
One of the first clearly identified and most widespread epigenetic modifications is called DNA methylation. This process involves attaching a small chemical (methyl molecule) tag to the DNA sequence.xv Certain patterns of DNA methylation can turn off gene expression and are essential for proper human development.xvi
Fetal DNA methylation can be influenced by the maternal environment, leading to lifelong health implications for the child. For example, one study demonstrated that elevated maternal blood sugar levels seen in gestational diabetes are associated with demethylation of the fetal gene that codes for the hormone leptin.xvii Leptin is a hormone that helps maintain a healthy weight by regulating body fat.xviii Demethylation of the fetal leptin gene related to elevated maternal blood glucose may affect the way that child later responds to leptin and can thus impact risk of obesity later in life.xix
Modifications to histone proteins
A second common epigenetic alteration is modifications to histone proteins, such as histone acetylation. Acetylation involves adding a different molecular tag, called an acetyl group.xx
Histones are proteins that help organize and package DNA in the cell. To organize and compact DNA, the DNA helix wraps around histone proteins, forming a structure called chromatin. Acetyl tags are added or removed from specific regions of histones in a process called acetylation or deacetylation, respectively. These modifications impact the DNA's compaction, making it easier or harder to read the genetic instructions.xxi
Some cell and mouse studies have suggested that maternal alcohol consumption during pregnancy may lead to alterations in histone acetylation in the fetal lungs, heart, and other tissues.xxii,xxiii,xxiv While more studies are needed to further determine the exact relevance of these epigenetic changes, these early investigations suggest that some of the negative impacts seen in fetal alcohol syndrome may be associated with histone acetylation changes.xxv
Non-coding RNA
Another set of epigenetic alterations are thought to develop from non-coding RNA segments, or RNA sequences that are transcribed but are not translated into proteins.xxvi These non-coding RNAs modify enzymes which change chromatin and alter gene expression.xxvii
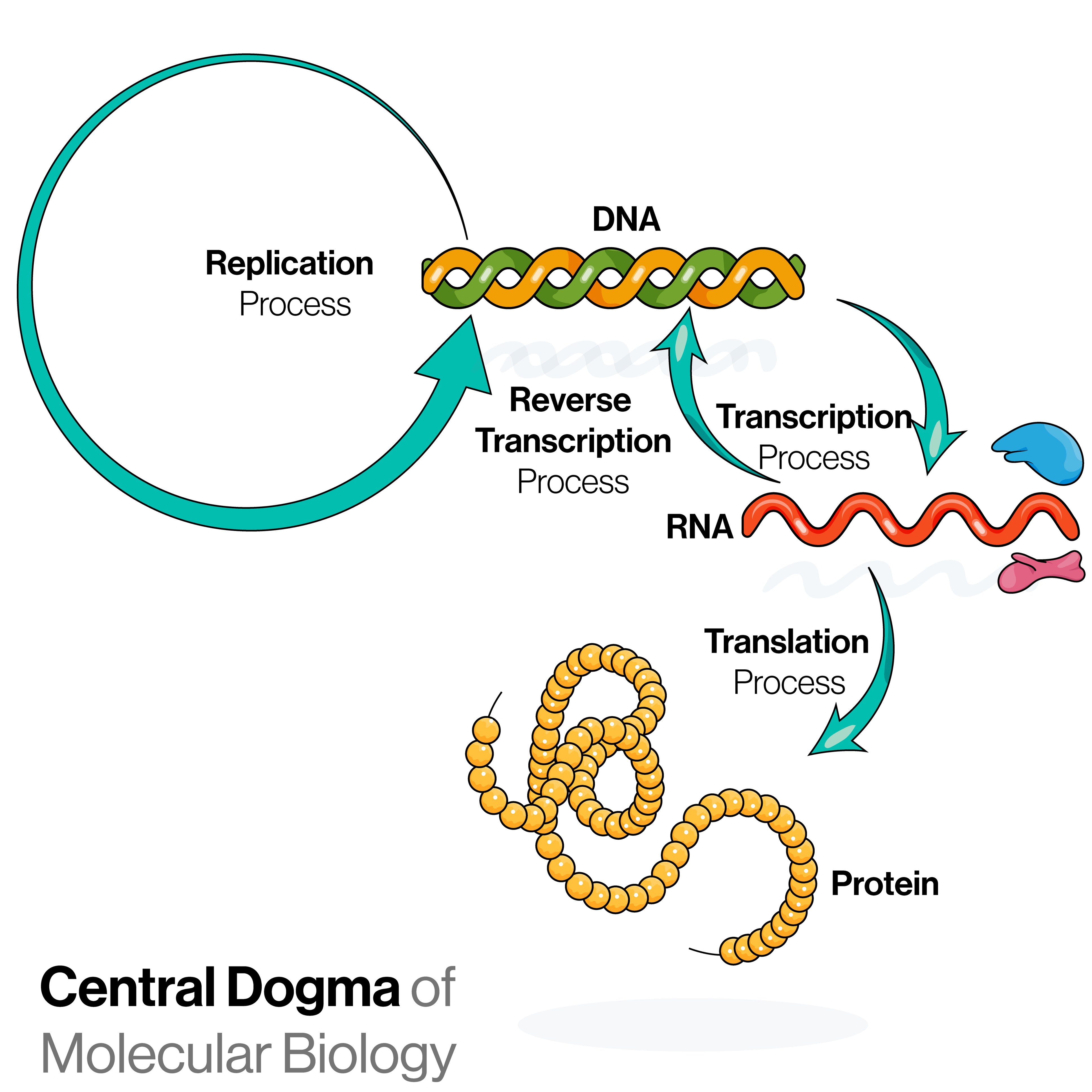
What is gene expression?
When a gene is expressed, it means that it is used as an instruction to make ribonucleic acid (RNA), which can then be translated to proteins (Figure 2). The proteins dictate the structure and function of the cells and tissues in the body.
In simplified terms, when a gene is transcribed and translated, we say the gene is “expressed” (Figure 2). Conversely, some genes can be “turned off” so that they do not get expressed, which means they will not be used to make proteins.
Epigenetic remodeling during fetal development
Soon after fertilization, the initial phases of embryonic development involve a significant episode of epigenetic remodeling, during which most of the epigenetic patterns carried by the parental DNA within the embryo are erased and reprogrammed.xxviii As the embryo goes on to develop from the early two-cell stage to the blastocyst stage, its genetic material changes shape. The folding and organization of the chromatin structure of the embryonic DNA transforms, allowing for more epigenetic changes to occur.xxix
Accurate timing and precision in these processes are crucial to avoid developmental issues or harm to the embryo. If the embryo’s epigenome is not established correctly, it could result in faulty activation of the embryonic genome. This short-but-crucial phase is vulnerable to influences from the mother’s uterus and the environment in which she lives.xxx
It is worth noting that not all parental epigenetic marks are wiped clean during the remodeling process described above. In the human genetic code, each gene has two versions — one from the sperm cell (paternal) and one from the oocyte (maternal). When the sperm and egg cells mature, some of the genes are marked with unique epigenetic tags, indicating them as either from the sperm or the egg, a process known as genomic imprinting.xxxi Such genes are referred to as “imprinted genes.”
Imprinted genes keep their epigenetic programming during and after the reprogramming period when most of the other epigenetic alterations are wiped clean.xxxii With imprinting, only one of the gene copies inherited is marked to be active (meaning it will be expressed). It creates a "parental signature" on certain genes, meaning that only the maternal or paternal copy will get expressed, not both. If there are changes to these markings, embryo development may be negatively impacted.xxxiii Approximately 99 percent of genes are not imprinted, and in most cases both maternal and paternal copies of the gene get expressed.
Epigenetic effects on developing embryos and fetuses
Some of the first key insights into fetal environmental exposure and future health come from the Dutch Hunger Winter study. This investigation, conducted after World War II, delved into the lasting health effects of exposure to famine during pregnancy in the Netherlands in 1944 to 1945xxxiv and illustrated the potential long-term effects of epigenetic modifications in utero.
Researchers in this study found that even 60 years later, individuals who were born right after the famine had significantly less DNA methylation of certain genes compared to their unexposed sex-matched siblings.xxxv It was particularly true for individuals who had been exposed to famine in the first 10 weeks of their fetal development.xxxvi Furthermore, these exposed subjects had an increased risk of health issues such as cardiovascular disease, diabetes, obesity, and psychiatric illness later in life.xxxvii
The implication is that epigenetic changes brought on by the maternal environment (e.g., lack of nutrition and stress) can have a lasting impact on future health. The Dutch Hunger study revolutionized our understanding of epigenetics, highlighting how environmental factors during crucial developmental periods can impact long-term health and even affect future generations.xxxviii
The Dutch Hunger Winter Study also indicated that there are particularly important times in the early stages of embryo growth when the epigenome is highly responsive to environmental factors. Data from this study suggests the first 10 weeks of embryo and fetal development are particularly crucial.xxxix During this time, it is thought that the embryo is particularly susceptible to epigenetic changes as it is undergoing epigenetic reprogramming, developing organized cell layers, and forming organs.xl, xli,xlii Factors such as nutrition, exposure to toxins, stress, and specific medications can impact how the epigenome develops during these crucial times (Figure 3). Research highlights the need to know when these critical periods occur in embryonic growth in order to find ways to improve fetal health and avoid potential problems.xliii
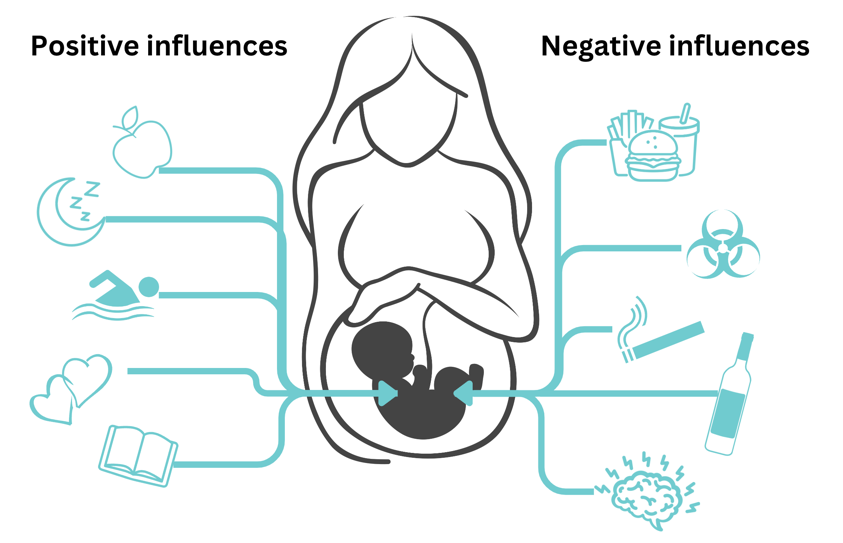
Beyond prenatal stages, recipient parents continue to impact epigenetics of their donor-conceived children throughout childhood and adolescence. The child’s environment, including positive experiences, such as supported learning and healthy relationships, help shape the child’s epigenome, which can influence gene expression into adulthood.
Epigenetics and donor eggs (oocytes) or donor embryos
Epigenetic modifications during fetal development occur whether the oocyte was derived from the intended mother (meaning she used her own egg), or whether it was a donor egg. If a donor egg is used to achieve a pregnancy, the genetic code inherited by the resulting child comes entirely from a mix of the egg donor and sperm. In other words, 50 percent of the DNA will come from the donor egg, and 50 percent of the DNA will come from the sperm. Thus, donor-conceived children do not inherit any genes (DNA) from the person giving birth (usually the intended mother).
However, the environment within the uterus of the mother carrying the fetus is very important to fetal development. A healthy intrauterine environment provides the fetus with nutrients, oxygen, and building blocks to grow, and also influences the epigenome of the offspring. So, even though an egg donor–conceived child does not share DNA with their mother, the birthing mother influences the child’s gene expression through epigenetic effects.xliv
Epigenetics involves the interplay between inherited genetic information and environmental factors that can modify gene expression.xlv The overwhelming majority of epigenetic modifications carried by the parental (egg and sperm) genome are globally reset during epigenetic reprogramming, which occurs during early embryonic development.xlvi In other words, the epigenetic marks are erased across the genome and new ones will be set. Imprinted genes (about 1 percent of genes) are an exception to this rule as their parental epigenetic signature is not erased.xlvii
In the context of donor eggs, almost all of the epigenetic information from the egg donor will be removed and then reprogrammed during embryonic and fetal development. Some of the epigenetic reprogramming may be influenced by the environment created within the uterus of the recipient mother.xlviii, xlix While the embryo resulting from the donor egg and sperm may carry some residual epigenetic marks from the egg donor and sperm, a large influence on the developing embryo's epigenetic profile comes from the recipient mother carrying the embryo/fetus.l
Following birth, parents continue to provide an environment that will influence epigenetic changes throughout childhood and adolescence.
Overall, it is important to understand that donor-conceived children will not directly inherit genes from the intended mother or the person giving birth to them. However, this individual has an extremely important role in shaping the donor-conceived child, both pre- and post-natally, starting from embryonic development. Epigenetics is just one way in which this impact is manifested.
Fertility treatments and epigenetics
Advanced reproductive technology (ART) procedures such as in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) involve manipulating sperm and oocytes outside of the body to develop a fertilized embryo, which is grown for several days before it is placed into the uterus. Therefore, the embryo is outside the body during at least part of the critical period of epigenetic reprogramming. It is unclear what, if any, effect this laboratory environment has on the reprogramming process.li While studies demonstrate that ART is associated with higher rates of fetal and maternal complications such as low birth weight, placental anomalies, and pre-eclampsia, the evidence does not link these outcomes to alterations in fetal epigenetics.lii
Although it is theoretically possible that ART impacts the fetal epigenome, there is currently a lack of consistent evidence to support this theory. Studying potential impacts is limited due to the diverse nature of ART procedures, discrepancies in tissue samples, heterogeneity of the epigenome, and variations in detection techniques.liii Furthermore, many variables such as maternal age, underlying causes of infertility, and specific ART methods are often not detailed in the studies.
A 2024 metanalysis of genome-wide studies that assessed epigenetic alterations during embryonic development did not conclusively identify widespread or consistent effects of ART on global methylation patterns.liv Recall that DNA methylation is a type of epigenetic modification (discussed above).
Controlled ovarian stimulation (COS) during IVF has been previously linked to changes in DNA methylation patterns during the maturation of oocytes. Research suggested that the heightened hormonal environment during COS could affect the formation of DNA methylation marks, potentially influencing gene regulation in the developing embryo.lv A meta-analysis conducted in 2022, covering 12 studies examining genome methylation patterns, indicated that offspring resulting from ART may have different methylation patterns compared to offspring from natural conception.lvi However, the conclusions are subject to debate due to the diversity among the studies.lvii
Additionally, a 2019 study on a distinctive group of individuals conceived through ART, with blood collected at birth and in adulthood (between 22 and 35 years old), found that later in adulthood there was no evidence of changes in DNA methylation patterns compared to individuals conceived without ART.lviii
Another potential impact on epigenetics is a reported increased risk of imprinting disorders in embryos created using ART, such as IVF with ICSI. The research findings are inconsistent across various studies, but some studies suggest a slightly elevated risk for imprinting disorders in ART-conceived individuals.lix,lx,lxi These risks are still exceptionally low.lxii,lxiii
It is also important to note that any potential effects of IVF on epigenetics discussed in this section are not specific to the use of donor eggs. They apply to the use of an individual’s own eggs, or use of donor embryos.
What traits can a birthing mother pass on to her donor-conceived child?
In the case of donor egg– or donor embryo–conceived children, the traits a donor egg recipient can pass on are nuanced. While physical similarities may arise from donor selection based on shared characteristics, there is no scientific evidence that heritable traits such as eye color, hair color, or height potential, among others, can be transferred from the donor egg recipient carrying the baby. Often the donor-conceived child will resemble the intended parent based on selecting a donor with similar characteristics. For some parents, this resemblance is important; for others it has no importance. All approaches are valid. Resemblance may also result from shared mannerisms, shared qualities, and/or the influence of the parenting environment, rather than genetic inheritance.
While the donor egg recipient does not share their children’s genetic code, they do influence embryo and fetal growth.lxiv As discussed above, mechanisms such as epigenetics and the overall health of the uterine environment contribute significantly to shaping the child’s development. Epigenetics highlights how environmental factors can impact gene expression, potentially influencing the future child's health and well-being.lxv
Conclusion
The interplay of genetics and environment in the context of epigenetics and donor-conceived children is complex. While physical traits are not directly inherited from the donor egg recipient, their role in shaping development through epigenetic mechanisms is one way in which they contribute to the child’s development. Using donor eggs or donor embryos can be a sensitive issue, and it is important to appreciate that a parent’s contribution to their child’s development extends vastly beyond the child’s DNA sequence.
i Osman, E. K., et al. (2018). Oocyte and Embryo Manipulation and Epigenetics. Seminars in Reproductive Medicine, 36(03/04), e1–e9. https://doi.org/10.1055/s-0039-1688801
ii Dvoran, M., et al. (2022). An Interplay between Epigenetics and Translation in Oocyte Maturation and Embryo Development: Assisted Reproduction Perspective. Biomedicines, 10(7), 1689–1689. https://doi.org/10.3390/biomedicines10071689
iii Dvoran, M., et al. (2022). An Interplay between Epigenetics and Translation in Oocyte Maturation and Embryo Development: Assisted Reproduction Perspective. Biomedicines, 10(7), 1689–1689. https://doi.org/10.3390/biomedicines10071689
iv Lei Cao-Lei, S., et al. (2020). Prenatal stress and epigenetics. Neuroscience & Biobehavioral Reviews, 117, 198–210. https://doi.org/10.1016/j.neubiorev.2017.05.016
v Orton, S. M., Millis, K., & Choate, P. (2023). Epigenetics of Trauma Transmission and Fetal Alcohol Spectrum Disorder: What Does the Evidence Support? International Journal of Environmental Research and Public Health, 20(17), 6706–6706. https://doi.org/10.3390/ijerph20176706
vi Berntsen, S., et al. (2019). The health of children conceived by ART: “the chicken or the egg?” Human Reproduction Update, 25(2), 137–158. https://doi.org/10.1093/humupd/dmz001
vii Berntsen, S., et al. (2019). The health of children conceived by ART: “the chicken or the egg?” Human Reproduction Update, 25(2), 137–158. https://doi.org/10.1093/humupd/dmz001
viii Lei Cao-Lei, S., et al. (2020). Prenatal stress and epigenetics. Neuroscience & Biobehavioral Reviews, 117, 198–210. https://doi.org/10.1016/j.neubiorev.2017.05.016
ix Epigenome. (2023). Genome.gov. https://www.genome.gov/genetics-glossary/Epigenome
x Epigenome. (2023). Genome.gov. https://www.genome.gov/genetics-glossary/Epigenome
xi Jaenisch, R., & Bird, A. (2003). Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics, 33(S3), 245–254. https://doi.org/10.1038/ng1089
xii Schuebel, K. E., et al. (2016). Making Sense of Epigenetics. The International Journal of Neuropsychopharmacology, 19(11), pyw058–pyw058. https://doi.org/10.1093/ijnp/pyw058
xiii Schuebel, K. E., Miri Gitik, Domschke, K., & Goldman, D. (2016). Making Sense of Epigenetics. The International Journal of Neuropsychopharmacology, 19(11), pyw058–pyw058. https://doi.org/10.1093/ijnp/pyw058
xiv Jaenisch, R., & Bird, A. (2003). Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics, 33(S3), 245–254. https://doi.org/10.1038/ng1089
xv Lei Cao-Lei, S., et al. (2020). Prenatal stress and epigenetics. Neuroscience & Biobehavioral Reviews, 117, 198–210. https://doi.org/10.1016/j.neubiorev.2017.05.016
xvi Borchiellini, M., et al. (2019). The Bright and Dark Side of DNA Methylation: A Matter of Balance. Cells, 8(10), 1243–1243. https://doi.org/10.3390/cells8101243
xiiv Sletner et al.. (2021). Maternal Glucose and LDL-Cholesterol Levels Are Related to Placental Leptin Gene Methylation, and, Together With Nutritional Factors, Largely Explain a Higher Methylation Level Among Ethnic South Asians. Frontiers in Endocrinology, 12. https://doi.org/10.3389/fendo.2021.809916
xviii Obradović et al. (2021). Leptin and Obesity: Role and Clinical Implication. Frontiers in Endocrinology, 12. https://doi.org/10.3389/fendo.2021.585887
xix Sletner et al. (2021). Maternal Glucose and LDL-Cholesterol Levels Are Related to Placental Leptin Gene Methylation, and, Together With Nutritional Factors, Largely Explain a Higher Methylation Level Among Ethnic South Asians. Frontiers in Endocrinology, 12. https://doi.org/10.3389/fendo.2021.809916
xx Kurdistani, S. K., et al. (2004). Mapping Global Histone Acetylation Patterns to Gene Expression. Cell, 117(6), 721–733. https://doi.org/10.1016/j.cell.2004.05.023
xxi Kurdistani, S. K., et al. (2004). Mapping Global Histone Acetylation Patterns to Gene Expression. Cell, 117(6), 721–733. https://doi.org/10.1016/j.cell.2004.05.023
xxii Wang, X., Prasra Gomutputra, Wolgemuth, D. J., & Baxi, L. (2010). Acute Alcohol Exposure Induces Apoptosis and Increases Histone H3K9/18 Acetylation in the Mid-Gestation Mouse Lung. Reproductive Sciences, 17(4), 384–390. https://doi.org/10.1177/1933719109356984
xxiii Mandal, C., et al. (2017). In Utero Alcohol Exposure and the Alteration of Histone Marks in the Developing Fetus: An Epigenetic Phenomenon of Maternal Drinking. International Journal of Biological Sciences, 13(9), 1100–1108. https://doi.org/10.7150/ijbs.21047
xxiv Mandal, C., et al. (2017). In Utero Alcohol Exposure and the Alteration of Histone Marks in the Developing Fetus: An Epigenetic Phenomenon of Maternal Drinking. International Journal of Biological Sciences, 13(9), 1100–1108. https://doi.org/10.7150/ijbs.21047
xxv Mandal, C., et al. (2017). In Utero Alcohol Exposure and the Alteration of Histone Marks in the Developing Fetus: An Epigenetic Phenomenon of Maternal Drinking. International Journal of Biological Sciences, 13(9), 1100–1108. https://doi.org/10.7150/ijbs.21047
xxvi Statello, L., et al. (2020). Gene regulation by long non-coding RNAs and its biological functions. Nature Reviews Molecular Cell Biology, 22(2), 96–118. https://doi.org/10.1038/s41580-020-00315-9
xxvii Zhang, G., & Pradhan, S. (2014). Mammalian epigenetic mechanisms. IUBMB Life, 66(4), 240–256. https://doi.org/10.1002/iub.1264
xxviii Smith, Z. D., et al. (2012). A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature, 484(7394), 339–344. https://doi.org/10.1038/nature10960
xxix Wilkinson, A. L., et al. (2023). Epigenetic regulation of early human embryo development. Cell Stem Cell, 30(12), 1569–1584. https://doi.org/10.1016/j.stem.2023.09.010
xxx Xia, W., & Xie, W. (2020). Rebooting the Epigenomes during Mammalian Early Embryogenesis. Stem Cell Reports, 15(6), 1158–1175. https://doi.org/10.1016/j.stemcr.2020.09.005
xxxi Monk, D., et al. (2019). Genomic imprinting disorders: lessons on how genome, epigenome and environment interact. Nature Reviews Genetics, 20(4), 235–248. https://doi.org/10.1038/s41576-018-0092-0
xxxii Monk, D., et al. (2019). Genomic imprinting disorders: lessons on how genome, epigenome and environment interact. Nature Reviews Genetics, 20(4), 235–248. https://doi.org/10.1038/s41576-018-0092-0
xxxiii Bajrami, E., & Мирко Спироски. (2016). Genomic Imprinting. Open Access Macedonian Journal of Medical Sciences, 4(1), 181–184. https://doi.org/10.3889/oamjms.2016.028
xxxiv Heijmans, B. T., et al. (2008). Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences of the United States of America, 105(44), 17046–17049. https://doi.org/10.1073/pnas.0806560105
xxxv Heijmans, B. T., et al. (2008). Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences of the United States of America, 105(44), 17046–17049. https://doi.org/10.1073/pnas.0806560105
xxxvi Tobi, E. W., et al. (2015). Early gestation as the critical time-window for changes in the prenatal environment to affect the adult human blood methylome. International Journal of Epidemiology, 44(4), 1211–1223. https://doi.org/10.1093/ije/dyv043
xxxvii Bleker, L. S., et al. (2021). Cohort profile: the Dutch famine birth cohort (DFBC)— a prospective birth cohort study in the Netherlands. BMJ Open, 11(3), e042078–e042078. https://doi.org/10.1136/bmjopen-2020-042078
xxxviii Heijmans, B. T., et al. (2008). Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proceedings of the National Academy of Sciences of the United States of America, 105(44), 17046–17049. https://doi.org/10.1073/pnas.0806560105
xxxix Tobi, E. W., et al. (2015). Early gestation as the critical time-window for changes in the prenatal environment to affect the adult human blood methylome. International Journal of Epidemiology, 44(4), 1211–1223. https://doi.org/10.1093/ije/dyv043
xl Kim, K.-C., et al. (2009). DNA methylation, an epigenetic mechanism connecting folate to healthy embryonic development and aging. The Journal of Nutritional Biochemistry, 20(12), 917–926. https://doi.org/10.1016/j.jnutbio.2009.06.008
xli Gabory, A., et al. (2011). Developmental programming and epigenetics. The American Journal of Clinical Nutrition, 94, S1943–S1952. https://doi.org/10.3945/ajcn.110.000927
xlii Gabory, A., et al. (2011). Developmental programming and epigenetics. The American Journal of Clinical Nutrition, 94, S1943–S1952. https://doi.org/10.3945/ajcn.110.000927
xliii Cai, S., et al. (2021). Nutritional Status Impacts Epigenetic Regulation in Early Embryo Development: A Scoping Review. Advances in Nutrition, 12(5), 1877–1892. https://doi.org/10.1093/advances/nmab038
xliv Wei, Y., et al. (2014). Environmental epigenetic inheritance through gametes and implications for human reproduction. Human Reproduction Update, 21(2), 194–208. https://doi.org/10.1093/humupd/dmu061
xlv Dvoran, M., et al. (2022). An Interplay between Epigenetics and Translation in Oocyte Maturation and Embryo Development: Assisted Reproduction Perspective. Biomedicines, 10(7), 1689–1689. https://doi.org/10.3390/biomedicines10071689
xlvi Berntsen, S., et al. (2019). The health of children conceived by ART: “the chicken or the egg?” Human Reproduction Update, 25(2), 137–158. https://doi.org/10.1093/humupd/dmz001
xlvii Litzky, J. F., & Marsit, C. J. (2019). Epigenetically regulated imprinted gene expression associated with IVF and infertility: possible influence of prenatal stress and depression. Journal of Assisted Reproduction and Genetics, 36(7), 1299–1313. https://doi.org/10.1007/s10815-019-01483-0
xlviii Berntsen, S., et al. (2019). The health of children conceived by ART: “the chicken or the egg?” Human Reproduction Update, 25(2), 137–158. https://doi.org/10.1093/humupd/dmz001
xlix Orton, S. M., & Choate, P. (2023). Epigenetics of Trauma Transmission and Fetal Alcohol Spectrum Disorder: What Does the Evidence Support? International Journal of Environmental Research and Public Health, 20(17), 6706–6706. https://doi.org/10.3390/ijerph20176706
l Dvoran, M., et al. (2022). An Interplay between Epigenetics and Translation in Oocyte Maturation and Embryo Development: Assisted Reproduction Perspective. Biomedicines, 10(7), 1689–1689. https://doi.org/10.3390/biomedicines10071689
li Schaub, A. M., et al. (2024). A systematic review of genome-wide analyses of methylation changes associated with assisted reproductive technologies in various tissues. Fertility and Sterility, 121(1), 80–94. https://doi.org/10.1016/j.fertnstert.2023.10.007
lii Schaub, A. M., et al. (2024). A systematic review of genome-wide analyses of methylation changes associated with assisted reproductive technologies in various tissues. Fertility and Sterility, 121(1), 80–94. https://doi.org/10.1016/j.fertnstert.2023.10.007
liii Schaub, A. M., et al. (2024). A systematic review of genome-wide analyses of methylation changes associated with assisted reproductive technologies in various tissues. Fertility and Sterility, 121(1), 80–94. https://doi.org/10.1016/j.fertnstert.2023.10.007
liv Schaub, A. M., et al. (2024). A systematic review of genome-wide analyses of methylation changes associated with assisted reproductive technologies in various tissues. Fertility and Sterility, 121(1), 80–94. https://doi.org/10.1016/j.fertnstert.2023.10.007
lv Market-Velker, B. A., et al. (2009). Dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner. Human Molecular Genetics, 19(1), 36–51. https://doi.org/10.1093/hmg/ddp465
lvi Cannarella, R., et al. (2022). DNA Methylation in Offspring Conceived after Assisted Reproductive Techniques: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(17), 5056–5056. https://doi.org/10.3390/jcm11175056
lvii Cannarella, R., et al. (2022). DNA Methylation in Offspring Conceived after Assisted Reproductive Techniques: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(17), 5056–5056. https://doi.org/10.3390/jcm11175056
lviii Novakovic, B., et al. (2019). Assisted reproductive technologies are associated with limited epigenetic variation at birth that largely resolves by adulthood. Nature Communications, 10(1). https://doi.org/10.1038/s41467-019-11929-9
lix Ahmadi, H., et al. (2023). Long-Term Effects of ART on the Health of the Offspring. International Journal of Molecular Sciences, 24(17), 13564–13564. https://doi.org/10.3390/ijms241713564
lx Henningsen, A. A., Gissler, M., Rasmussen, S., Opdahl, S., Wennerholm, U. B., Spangsmose, A. L., A Tiitinen, Bergh, C., Romundstad, L. B., H Laivuori, Forman, J. L., A Pinborg, & Ø Lidegaard. (2020). Imprinting disorders in children born after ART: a Nordic study from the CoNARTaS group. Human Reproduction, 35(5), 1178–1184. https://doi.org/10.1093/humrep/deaa039
lxi Sciorio, R. and Esteves, S.C. (2022). Contemporary Use of ICSI and Epigenetic Risks to Future Generations. Journal of Clinical Medicine, 11(8), pp.2135–2135. https://doi.org/10.3390/jcm11082135
lxii Ahmadi, H., et al. (2023). Long-Term Effects of ART on the Health of the Offspring. International Journal of Molecular Sciences, 24(17), 13564–13564. https://doi.org/10.3390/ijms241713564\
lxiii Henningsen, A. A., Gissler, M., Rasmussen, S., Opdahl, S., Wennerholm, U. B., Spangsmose, A. L., A Tiitinen, Bergh, C., Romundstad, L. B., H Laivuori, Forman, J. L., A Pinborg, & Ø Lidegaard. (2020). Imprinting disorders in children born after ART: a Nordic study from the CoNARTaS group. Human Reproduction, 35(5), 1178–1184. https://doi.org/10.1093/humrep/deaa039
lxiv Kitsiou-Tzeli, S., & Tzetis, M. (2017). Maternal epigenetics and fetal and neonatal growth. Current Opinion in Endocrinology, Diabetes and Obesity, 24(1), 43–46. https://doi.org/10.1097/med.0000000000000305
lxv Kitsiou-Tzeli, S., & Tzetis, M. (2017). Maternal epigenetics and fetal and neonatal growth. Current Opinion in Endocrinology, Diabetes and Obesity, 24(1), 43–46. https://doi.org/10.1097/med.0000000000000305



